MULTI-SCALE STUDY OF NON-CODING DNA IN LEUKEMIA
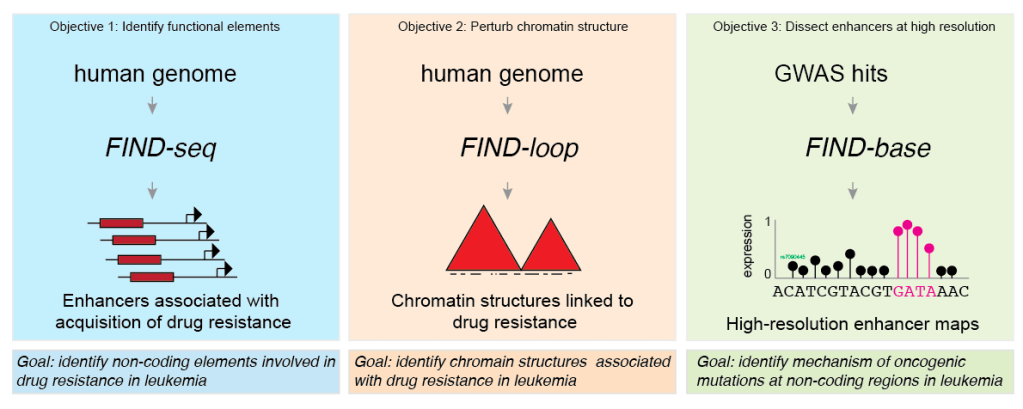
Functional non-coding regions such as enhancers and insulators play an essential role in the regulation of cell type-specific gene expression programs, and mutations at non-coding elements can drive observable phenotypic changes comparable to those driven by mutations at coding sequences (see Seruggia et al. 2015; Seruggia et al. 2020). However, due to lack of appropriate technology, just a limited number of disease-related regulatory sequences have been described, leaving many potential targets of therapy to be discovered. For this reasons, our goal is to investigate the contribution of non-coding sequences and 3D genome structure in cancer. We focus on leukemia predisposition and on development of drug resistance. What is the role of enhancers in the acquisition of drug resistance? How chromatin topology affects gene expression in leukemia? What is the effect of sequence variation at enhancers whose mutation is associated with leukemia? We use epi/genome editing (CRISPR, CRISPRi, CRISPRa), chromatin profiling (ChIP-seq, Cut and Run, ATAC-seq) and computational biology to answer these questions. See our preprint on dense base editing at enhancers.
TFIID/SAGA COMPONENTS AS TARGETS FOR MYC-DRIVEN PEDIATRIC CANCER
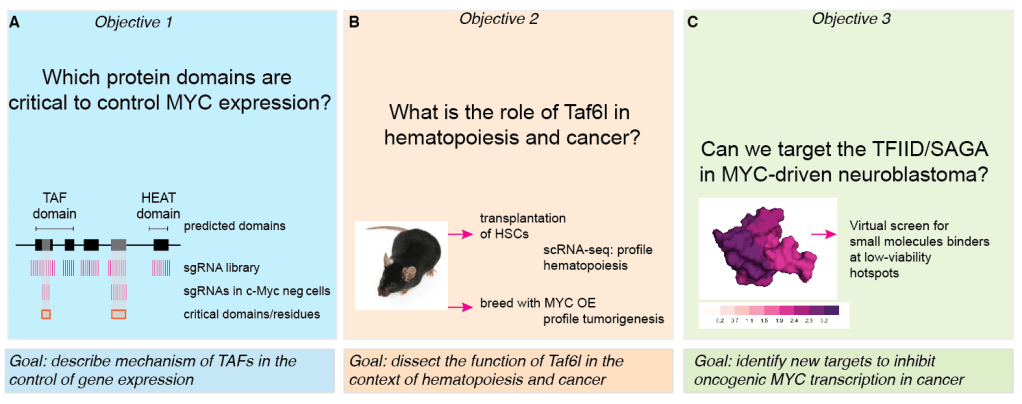
We previously reported a connection between two chromatin modifiers of the SAGA complex and self-renewal in mouse embryonic stem cells (see Seruggia et al 2019). We discovered that loss of Taf5l and Taf6l in mESCs results in a dramatic reduction in c-Myc expression at the RNA and protein levels. However, the functions of SAGA CORE components in mESCs and other cell types, as well as their mechanism of action, remain unexplored. Can we harness vulnerabilities within the TFIID and SAGA members to treat leukemia and other types of cancer? We use mouse models, genomics and genome editing to answer these questions.
MOUSE MODELS
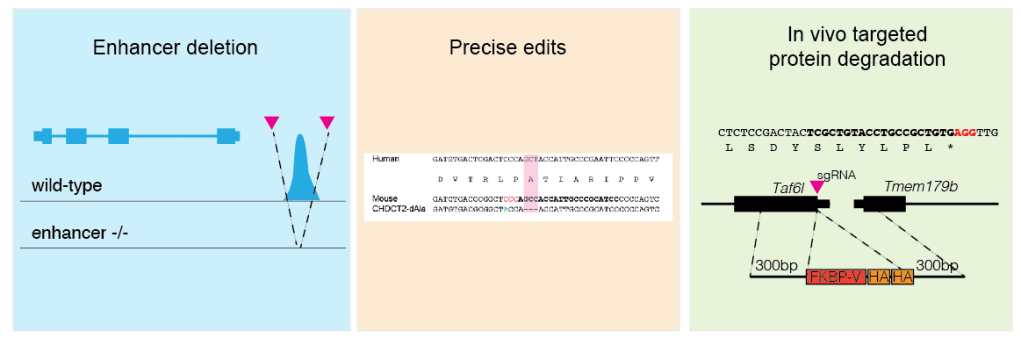
We are interested in developing new animal models to study hematopoiesis and leukemia development. In the lab, we design and prepare CRISPR reagents suitable to treat mouse embryos at the 1-cell stage. We have successfully targeted regulatory sequences (see Seruggia et al. 2015, Seruggia et al. 2020, Singh et al. 2022), introduced specific point mutations (see Sher et al. 2019) and generated degrons or conditional alleles for our favorite genes (see Mehta et al. 2022; Manieri et al. 2023).
